Abstract
Introduction: Hypomethylating agents (HMA) have shown significant therapeutic efficacy in the treatment of patients with MDS and AML. Although it has been suggested, that HMA act through re-activation of tumor-suppressor genes, the exact mechanism of their action has not been completely elucidated yet. The aim of our study was to identify predictors of outcome for patients treated with azacytidine (AZA).
Patients and Methods: Eighteen patients with high risk MDS (RAEB-II) and oligoblastic AML (20-30% bone marrow blasts) were included in this study. Patients were not eligible for intensive induction chemotherapy due to age, or presence of comorbidities. All patients were selected for treatment with AZA.
NK-cytotoxicity against K562, Rajii, and the percentage of various immune regulatory subpopulations in the peripheral blood (PB) was measured after 3-6 cycles of AZA. A previous short exposure to AZA was required with the aim: 1) to examine the effect of AZA on these populations, and 2) to identify biomarkers with the potential to predict outcome after treatment with AZA.
NK-cytotoxicity against K562 and Rajii was tested by using a flow cytometry based assay as previously described (1).
Quantitative estimation of immune regulatory subpopulations in PB of patients was performed by using flow cytometry. The number of myeloid derived suppressor cells (MDSCs) (CD33+/CD11b+/CD14-/HLADRlow/-) was expressed as percentage among CD45+ PB monocytes. The number of Tregs (CD3+/CD4+/CD25+/Foxp3+) was expressed as percentage among CD3+/CD4+ PB lymphocytes. The number of double negative TCRαβ (CD3+/TCRαβ+/CD4-/CD8-) was expressed as percentage among PB CD3+ lymphocytes.
Comparison between groups was performed by using Mann-Whitney test for continuous variables. Receiver operating curve analysis (ROC) was performed with the aim to determine the ability of various parameters for the prediction of 2-year survival. Kaplan-Meier and log rank test were used for estimating the probability of overall survival (OS).
Results
NK cytotoxicity: The median NK-cytotoxicity against K562 of patients and healthy donors was 21.8% (95% CI, 7.5 - 32.6) and 41.8% (95% CI, 11.4 - 56.1). Patients with MDS/AML after a short exposure to azacytidine had a significantly reduced NK-cytotoxicity against K562 as compared with NK cytotoxic activity of healthy donors (p=0.01). NK cytotoxic activity against Rajii of both patients and healthy donors was very low to absent (data not shown).
Immune regulatory cells: The median MDSCs of patients and healthy donors was 0.41% (95% CI, 0.13 - 1.26) and 0.60% (95% CI, 0.30 - 1.94). There was no difference between MDSCs in the PB of patients and healthy donors. There was no difference in the number of Tregs and double negative TCRαβ cells between patients and donors (data not shown).
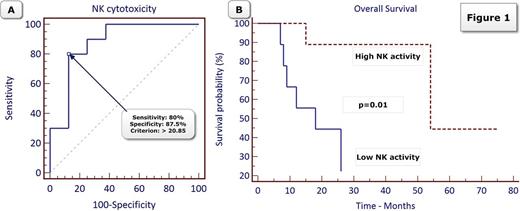
NK cytotoxicity is a predictor of overall survival in patients treated with AZA: The ability of various parameters including the PB percentage of MDSCs, Tregs, and double negative TCRαβ, as well as of NK cytotoxic activity to predict survival at 2 years after treatment with AZA was examined by using ROC analysis. NK cytotoxic activity was the only predictor of OS for patients treated with AZA (AUC=0.875, 95% CI, 0.635 - 0.979, p<0.0001). NK-cytotoxicity above 20.85% was estimated as the best cut off with sensitivity and specificity 80% and 87% in predicting 2-year survival (Figure 1A). In multivariate analysis, NK cytotoxicity above 20.85% (high NK-activity) was the only parameter statistically associated with significantly improved OS [HR=0.10, 95% CI, 0.01 - 0.90, p=0.041] (Figure 1B).
Conclusions: Our study showed that NK cytotoxicity is a predictor of the outcome for patients treated with AZA. Patients with high NK-activity after a short course of AZA had significantly improved OS as compared with patients with low NK-activity. The results of our study suggest that the therapeutic activity of AZA is at least partly mediated by an immunomodulatory effect.
References: 1) Irradiated mononuclear cells express significant in vitro cytotoxic activity: promise for in vivo clinical efficacy of irradiated mismatched donor lymphocytes infusion. Immunotherapy (2014) 6(4), 409-417
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal